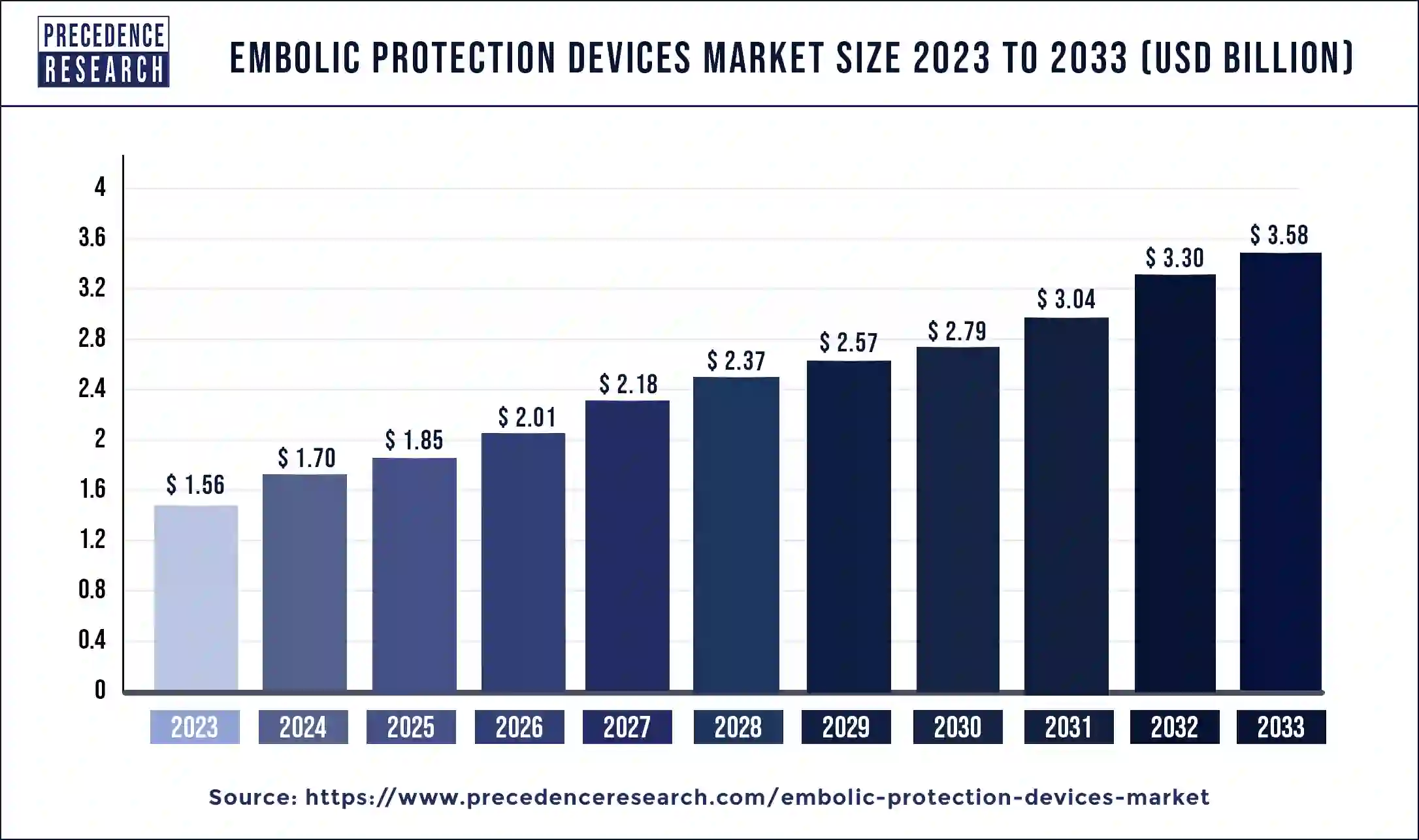
The global embolic protection devices market size was exhibited at USD 1.56 billion in 2023 and is anticipated to be expanding around USD 3.58 Billion By 2033, growing at a impressive CAGR of 8.64% from 2024 to 2033
Key Points
- The North America embolic protection devices market size accounted for USD 560 million in 2023 and is expected to attain around USD 1,310 million by 2033, poised to grow at a CAGR of 8.87% between 2024 and 2033.
- North America has held a major revenue share of 36% in 2023.
- Asia Pacific is expected to show rapid growth in the global market during the forecast period.
- By product, the distal occlusion systems segment held a significant share of the market in 2023.
- By product, the proximal occlusion systems segment is expected to grow rapidly in the market over the projected period.
- By application, the cardiovascular diseases segment has contributed more than 41% of revenue share in 2023.
- By application, the peripheral vascular diseases segment is expected to witness the fastest growth in the market over the forecast period.
Market Overview
The Embolic Protection Devices (EPDs) market encompasses devices designed to capture and remove debris that may be dislodged during cardiovascular procedures, particularly those involving the arteries. These devices are crucial for preventing complications such as strokes and other embolic events, which can occur when fragments of plaque or other materials are released into the bloodstream. EPDs are widely used in procedures like carotid artery stenting and percutaneous coronary interventions. The market for these devices is driven by the increasing prevalence of cardiovascular diseases, advancements in medical technology, and a growing awareness of the benefits of these devices.
Growth Factors
The primary growth factors for the EPD market include the rising incidence of cardiovascular diseases globally, attributed to an aging population, unhealthy lifestyles, and increasing prevalence of diabetes and hypertension. Technological advancements in the design and functionality of EPDs, which enhance their safety and efficacy, are also propelling market growth. Moreover, increasing adoption of minimally invasive surgical procedures, which require the use of EPDs, further boosts the market. Favorable reimbursement policies and increased healthcare expenditure in emerging economies are additional factors contributing to market expansion.
Embolic Protection Devices Market Scope
| Report Coverage | Details |
| Embolic Protection Devices Market Size in 2023 | USD 1.56 Billion |
| Embolic Protection Devices Market Size in 2024 | USD 1.70 Billion |
| Embolic Protection Devices Market Size by 2033 | USD 3.58 Billion |
| Embolic Protection Devices Market Growth Rate | CAGR of 8.64% from 2024 to 2033 |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Product, Application, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Key drivers of the EPD market include the growing prevalence of cardiovascular and neurovascular diseases, which necessitate the use of embolic protection during various procedures. The increasing preference for minimally invasive surgeries, which reduce recovery time and hospital stays, also drives demand for these devices. Continuous innovations and improvements in EPD technology enhance their effectiveness and safety, further encouraging their adoption. Additionally, supportive government policies and funding for cardiovascular disease management contribute to market growth.
Opportunities
There are significant opportunities for growth in the embolic protection devices market, particularly in emerging economies where healthcare infrastructure is rapidly developing. Increasing investment in healthcare by governments and private sectors in these regions can create new market opportunities. The development of next-generation EPDs that offer better patient outcomes and ease of use also presents opportunities for manufacturers. Moreover, expanding applications of EPDs in various surgical and interventional procedures beyond traditional cardiovascular interventions can broaden the market scope.
Challenges
Despite the positive outlook, the EPD market faces several challenges. High costs associated with these devices and related procedures can limit their accessibility, particularly in low and middle-income countries. Stringent regulatory requirements and the need for rigorous clinical trials to ensure safety and efficacy can also pose hurdles for market entry and expansion. Additionally, the risk of device-related complications and the need for skilled professionals to operate these devices effectively can impact their adoption. Overcoming these challenges requires continuous innovation, cost-effective solutions, and extensive training programs for healthcare professionals.
Region Insights
North America currently holds the largest share of the embolic protection devices market, driven by high healthcare expenditure, advanced healthcare infrastructure, and a significant number of cardiovascular procedures performed annually. Europe follows, with robust growth due to the increasing adoption of advanced medical technologies and a rising elderly population. The Asia-Pacific region is expected to witness the fastest growth during the forecast period. Factors such as improving healthcare infrastructure, increasing healthcare awareness, and a growing patient pool with cardiovascular diseases are driving this growth. Countries like China and India are significant contributors due to their large populations and rapid economic development.
Read Also: https://www.expresswebwire.com/self-adhesive-labels-market/
Embolic Protection Devices Market Recent Developments
- In January 2023, Cardinal Health Inc., a distinguished leader in healthcare services and medical products, embarked on a strategic collaboration with Palantir, a renowned technology company specializing in data integration and analysis. This innovative partnership marks a significant step forward in the realm of healthcare supply chain management.
- In February 2023, Medtronic plc announced the launch of its EnVeo™ drug-eluting stent (DES) for the treatment of patients with PAD.
- In March 2023, Terumo Corporation received a CE mark for its Zilver PTX™ DCB for the treatment of patients with PAD.
- In July 2022, Edwards Lifesciences Corporation announced the FDA approval of its Amplatzer™ Vascular Plug II for the treatment of patients with pulmonary arteriovenous malformations (PAVMs).
- In June 2022, Cook Medical announced the FDA approval of its NirMesh™ Vascular Occlusion Device for the treatment of patients with varicose veins.
- In May 2022, Johnson & Johnson Services, Inc. announced the FDA approval of its Embozene™ Microspheres for the treatment of patients with symptomatic cerebral arteriovenous malformations (AVMs).
- In October 2023, German cardiovascular medical device company Protembis secured US Food and Drug Administration (FDA) approval for its PROTEMBO Pivotal IDE Trial. The ProtEmbo System from Protembis is an intra-aortic filter device offering protection for the brain from embolic material released during a transcatheter aortic valve replacement (TAVR) procedure.
Embolic Protection Devices Market Companies
- Abbott Laboratories
- Allium Medical Solutions Ltd.
- Boston Scientific Corporation
- Cardinal Health Inc.
- Contego Medical LLC
- Innovative Cardiovascular Solutions LLC
- Edwards Lifesciences Corporation
- Medtronic Inc.
- Silk Road Medical Inc.
Segments Covered in the Report
By Product
- Distal Occlusion Filters
- Proximal Occlusion Filters
- Distal Filters
By Application
- Neurovascular Diseases
- Cardiovascular Diseases
- Peripheral Vascular Diseases
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.dailystatsnews.com/
Blog: https://www.dailytechbulletin.com/
