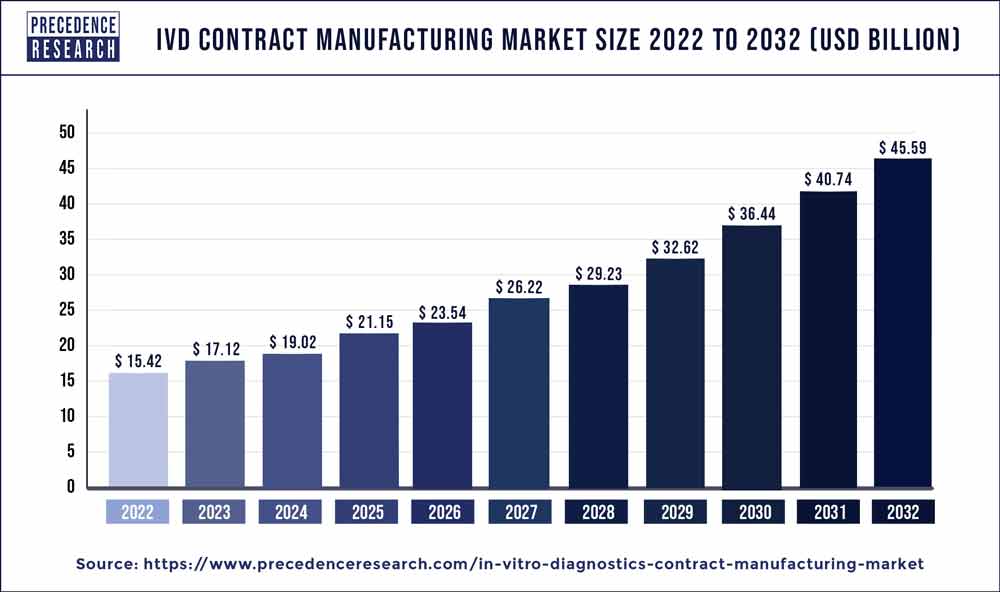
The IVD contract manufacturing market size is poised to grow by USD 45.59 billion by 2032 from USD 17.12 billion in 2023, exhibiting a CAGR of 11.50% during the forecast period 2023-2032.
Key Takeaways
- North America contributed more than 45% of revenue share in 2022.
- Asia Pacific is estimated to witness the fastest CAGR between 2023 and 2032.
- By device type, the IVD consumables segment has held the largest market share of 68% in 2022.
- By device type, the IVD equipment segment is anticipated to grow at a remarkable CAGR of 13.5% between 2023 and 2032.
- By technology, the immunoassay segment generated over 35% of revenue share in 2022.
- By technology, the molecular diagnostics segment is expected to expand at the fastest CAGR over the projected period.
- By service, the assay development segment generated over 45% of revenue share in 2022.
- By service, the manufacturing segment is expected to expand at the fastest CAGR over the projected period.
Precedence Research has conducted a comprehensive market study that provides valuable insights into the performance of the market during the forecast period. The study identifies significant trends that are shaping the growth of the IVD contract manufacturing market. In this recently published report, essential dynamics such as drivers, restraints, and opportunities are highlighted for both established market players and emerging participants involved in production and supply.
To begin with, the IVD contract manufacturing Market report features an executive summary that offers a concise overview of the marketplace. It outlines the key players and industry categories expected to have an impact on the market in the coming years. The executive summary provides an unbiased summary of the market.
Get a Sample Report: https://www.precedenceresearch.com/sample/3641
IVD Contract Manufacturing Market Scope
| Report Coverage | Details |
| Growth Rate from 2023 to 2032 | CAGR of 11.50% |
| Market Size in 2023 | USD 17.12 Billion |
| Market Size by 2032 | USD 45.59 Billion |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Segments Covered | By Device Type, By Technology, and By Service Type |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Read More: Inhalable Drugs Market Size To Gain USD 58.21 Bn by 2032
The empirical study on the global IVD contract manufacturing market primarily focuses on the drivers in subsequent sections. It demonstrates how changing demographics are projected to influence the supply and demand dynamics in the IVD contract manufacturing Market. Our market report for the IVD contract manufacturing market also delves into the significant rules and regulations that are likely to impact the future growth of this sector. Moreover, in order to comprehend the underlying demand factors, industry experts have provided insights into its fundamental origins.
IVD Contract Manufacturing Market Players
- Thermo Fisher Scientific Inc.
- Siemens Healthineers AG
- Danaher Corporation
- Roche Diagnostics
- Ortho Clinical Diagnostics
- Abbott Laboratories
- Bio-Rad Laboratories, Inc.
- Sysmex Corporation
- Quidel Corporation
- BioMérieux SA
- PerkinElmer Inc.
- DiaSorin S.p.A.
- Luminex Corporation
- Becton, Dickinson and Company (BD)
- Meridian Bioscience, Inc.
Data Sources and Methodology
To gather comprehensive insights on the Global IVD contract manufacturing Market, we relied on a range of data sources and followed a well-defined methodology. Our approach involved interactions with industry experts and key stakeholders across the market’s value chain, including management organizations, processing organizations, and analytics service providers.
We followed a rigorous data analysis process to ensure the quality and credibility of our research. The gathered information was carefully evaluated, and relevant quantitative data was subjected to statistical analysis. By employing robust analytical techniques, we were able to derive meaningful insights and present a comprehensive overview of the Global IVD contract manufacturing Market.
The most resonating, simple, genuine, and important causes because of which you must decide to buy the IVD contract manufacturing market report exclusively from precedence research
- The research report has been meticulously crafted to provide comprehensive knowledge on essential marketing strategies and a holistic understanding of crucial marketing plans spanning the forecasted period from 2023 to 2032.
Key Features of the Report:
- Comprehensive Coverage: The report extensively encompasses a detailed explanation of highly effective analytical marketing methods applicable to companies across all industry sectors.
- Decision-Making Enhancement: It outlines a concise overview of the decision-making process while highlighting key techniques to enhance it, ensuring favorable business outcomes in the future.
- Articulated R&D Approach: The report presents a well-defined approach to conducting research and development (R&D) activities, enabling accurate data acquisition on current and future marketing conditions.
Market Segmentation:
By Device Type
- IVD Equipment
- IVD Consumables
By Technology
- Immunoassay
- Clinical Chemistry
- Molecular Diagnostics
- Microbiology
- Hematology
- Coagulation & Hemostasis
- Others
By Service Type
- Manufacturing Services
- Assay Development Services
- Other Services
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Reasons to Consider Purchasing the Report:
- Enhance your market research capabilities by accessing this comprehensive and precise report on the global IVD contract manufacturing market.
- Gain a thorough understanding of the overall market landscape and be prepared to overcome challenges while ensuring robust growth.
- Benefit from in-depth research and analysis of the latest trends shaping the global IVD contract manufacturing market.
- Obtain detailed insights into evolving market trends, current and future technologies, and strategic approaches employed by key players in the global IVD contract manufacturing market.
- Receive valuable recommendations and guidance for both new entrants and established players seeking further market expansion.
- Discover not only the cutting-edge technological advancements in the global IVD contract manufacturing market but also the strategic plans of industry leaders.
Table of Content
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology (Premium Insights)
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on IVD Contract Manufacturing Market
5.1. COVID-19 Landscape: IVD Contract Manufacturing Industry Impact
5.2. COVID 19 – Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global IVD Contract Manufacturing Market, By Device Type
8.1. IVD Contract Manufacturing Market, by Device Type, 2023-2032
8.1.1 IVD Equipment
8.1.1.1. Market Revenue and Forecast (2020-2032)
8.1.2. IVD Consumables
8.1.2.1. Market Revenue and Forecast (2020-2032)
Chapter 9. Global IVD Contract Manufacturing Market, By Technology
9.1. IVD Contract Manufacturing Market, by Technology, 2023-2032
9.1.1. Immunoassay
9.1.1.1. Market Revenue and Forecast (2020-2032)
9.1.2. Clinical Chemistry
9.1.2.1. Market Revenue and Forecast (2020-2032)
9.1.3. Molecular Diagnostics
9.1.3.1. Market Revenue and Forecast (2020-2032)
9.1.4. Microbiology
9.1.4.1. Market Revenue and Forecast (2020-2032)
9.1.5. Hematology
9.1.5.1. Market Revenue and Forecast (2020-2032)
9.1.6. Coagulation & Hemostasis
9.1.6.1. Market Revenue and Forecast (2020-2032)
9.1.7. Others
9.1.7.1. Market Revenue and Forecast (2020-2032)
Chapter 10. Global IVD Contract Manufacturing Market, By Service Type
10.1. IVD Contract Manufacturing Market, by Service Type, 2023-2032
10.1.1. Manufacturing Services
10.1.1.1. Market Revenue and Forecast (2020-2032)
10.1.2. Assay Development Services
10.1.2.1. Market Revenue and Forecast (2020-2032)
10.1.3. Other Services
10.1.3.1. Market Revenue and Forecast (2020-2032)
Chapter 11. Global IVD Contract Manufacturing Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, by Device Type (2020-2032)
11.1.2. Market Revenue and Forecast, by Technology (2020-2032)
11.1.3. Market Revenue and Forecast, by Service Type (2020-2032)
11.1.4. U.S.
11.1.4.1. Market Revenue and Forecast, by Device Type (2020-2032)
11.1.4.2. Market Revenue and Forecast, by Technology (2020-2032)
11.1.4.3. Market Revenue and Forecast, by Service Type (2020-2032)
11.1.5. Rest of North America
11.1.5.1. Market Revenue and Forecast, by Device Type (2020-2032)
11.1.5.2. Market Revenue and Forecast, by Technology (2020-2032)
11.1.5.3. Market Revenue and Forecast, by Service Type (2020-2032)
11.2. Europe
11.2.1. Market Revenue and Forecast, by Device Type (2020-2032)
11.2.2. Market Revenue and Forecast, by Technology (2020-2032)
11.2.3. Market Revenue and Forecast, by Service Type (2020-2032)
11.2.4. UK
11.2.4.1. Market Revenue and Forecast, by Device Type (2020-2032)
11.2.4.2. Market Revenue and Forecast, by Technology (2020-2032)
11.2.4.3. Market Revenue and Forecast, by Service Type (2020-2032)
11.2.5. Germany
11.2.5.1. Market Revenue and Forecast, by Device Type (2020-2032)
11.2.5.2. Market Revenue and Forecast, by Technology (2020-2032)
11.2.5.3. Market Revenue and Forecast, by Service Type (2020-2032)
11.2.6. France
11.2.6.1. Market Revenue and Forecast, by Device Type (2020-2032)
11.2.6.2. Market Revenue and Forecast, by Technology (2020-2032)
11.2.6.3. Market Revenue and Forecast, by Service Type (2020-2032)
11.2.7. Rest of Europe
11.2.7.1. Market Revenue and Forecast, by Device Type (2020-2032)
11.2.7.2. Market Revenue and Forecast, by Technology (2020-2032)
11.2.7.3. Market Revenue and Forecast, by Service Type (2020-2032)
11.3. APAC
11.3.1. Market Revenue and Forecast, by Device Type (2020-2032)
11.3.2. Market Revenue and Forecast, by Technology (2020-2032)
11.3.3. Market Revenue and Forecast, by Service Type (2020-2032)
11.3.4. India
11.3.4.1. Market Revenue and Forecast, by Device Type (2020-2032)
11.3.4.2. Market Revenue and Forecast, by Technology (2020-2032)
11.3.4.3. Market Revenue and Forecast, by Service Type (2020-2032)
11.3.5. China
11.3.5.1. Market Revenue and Forecast, by Device Type (2020-2032)
11.3.5.2. Market Revenue and Forecast, by Technology (2020-2032)
11.3.5.3. Market Revenue and Forecast, by Service Type (2020-2032)
11.3.6. Japan
11.3.6.1. Market Revenue and Forecast, by Device Type (2020-2032)
11.3.6.2. Market Revenue and Forecast, by Technology (2020-2032)
11.3.6.3. Market Revenue and Forecast, by Service Type (2020-2032)
11.3.7. Rest of APAC
11.3.7.1. Market Revenue and Forecast, by Device Type (2020-2032)
11.3.7.2. Market Revenue and Forecast, by Technology (2020-2032)
11.3.7.3. Market Revenue and Forecast, by Service Type (2020-2032)
11.4. MEA
11.4.1. Market Revenue and Forecast, by Device Type (2020-2032)
11.4.2. Market Revenue and Forecast, by Technology (2020-2032)
11.4.3. Market Revenue and Forecast, by Service Type (2020-2032)
11.4.4. GCC
11.4.4.1. Market Revenue and Forecast, by Device Type (2020-2032)
11.4.4.2. Market Revenue and Forecast, by Technology (2020-2032)
11.4.4.3. Market Revenue and Forecast, by Service Type (2020-2032)
11.4.5. North Africa
11.4.5.1. Market Revenue and Forecast, by Device Type (2020-2032)
11.4.5.2. Market Revenue and Forecast, by Technology (2020-2032)
11.4.5.3. Market Revenue and Forecast, by Service Type (2020-2032)
11.4.6. South Africa
11.4.6.1. Market Revenue and Forecast, by Device Type (2020-2032)
11.4.6.2. Market Revenue and Forecast, by Technology (2020-2032)
11.4.6.3. Market Revenue and Forecast, by Service Type (2020-2032)
11.4.7. Rest of MEA
11.4.7.1. Market Revenue and Forecast, by Device Type (2020-2032)
11.4.7.2. Market Revenue and Forecast, by Technology (2020-2032)
11.4.7.3. Market Revenue and Forecast, by Service Type (2020-2032)
11.5. Latin America
11.5.1. Market Revenue and Forecast, by Device Type (2020-2032)
11.5.2. Market Revenue and Forecast, by Technology (2020-2032)
11.5.3. Market Revenue and Forecast, by Service Type (2020-2032)
11.5.4. Brazil
11.5.4.1. Market Revenue and Forecast, by Device Type (2020-2032)
11.5.4.2. Market Revenue and Forecast, by Technology (2020-2032)
11.5.4.3. Market Revenue and Forecast, by Service Type (2020-2032)
11.5.5. Rest of LATAM
11.5.5.1. Market Revenue and Forecast, by Device Type (2020-2032)
11.5.5.2. Market Revenue and Forecast, by Technology (2020-2032)
11.5.5.3. Market Revenue and Forecast, by Service Type (2020-2032)
Chapter 12. Company Profiles
12.1. Thermo Fisher Scientific Inc.
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. Siemens Healthineers AG
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. Danaher Corporation
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. Roche Diagnostics
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. Ortho Clinical Diagnostics
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. Abbott Laboratories
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. Bio-Rad Laboratories, Inc.
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. Sysmex Corporation
12.8.1. Company Overview
12.8.2. Product Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
12.9. Quidel Corporation
12.9.1. Company Overview
12.9.2. Product Offerings
12.9.3. Financial Performance
12.9.4. Recent Initiatives
12.10. BioMérieux SA
12.10.1. Company Overview
12.10.2. Product Offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
Unlocking Market Insights through Data Excellence
The “Precedence Statistics” flexible dashboard is a powerful tool that offers real-time news updates, economic and market forecasts, and customizable reports. It can be configured to support a wide range of analysis styles and strategic planning needs. This tool empowers users to stay informed and make data-driven decisions in various scenarios, making it a valuable asset for businesses and professionals looking to stay ahead in today’s dynamic and data-driven world.
Access our Premium Real Time Data Intelligence Tool, Visit: www.precedencestatistics.com
Precedence Statistics – Empowering Your Data Insights
Contact Us
Precedence Research
Apt 1408 1785 Riverside Drive Ottawa, ON, K1G 3T7, Canada
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
