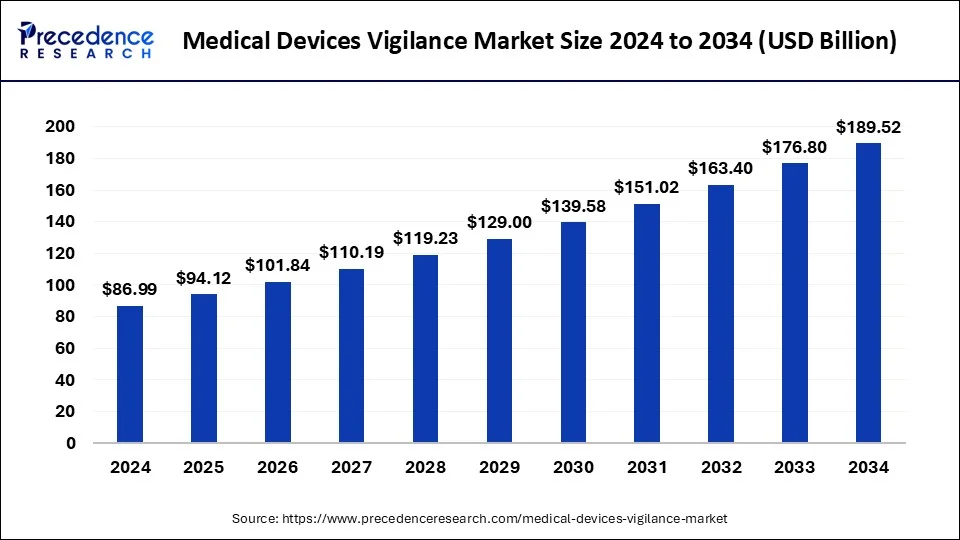
The medical devices vigilance market involves monitoring, reporting, and managing adverse events and safety concerns related to medical devices. This includes evaluating device performance, potential risks, and any issues reported by healthcare professionals or patients. The market aims to ensure the safety and efficacy of medical devices, protecting public health and maintaining regulatory compliance.
Get a Sample: https://www.precedenceresearch.com/sample/4120
Growth Factors
The growth of the medical devices vigilance market is primarily driven by the increasing adoption of medical devices worldwide, the rise in chronic diseases, and the growing aging population. Additionally, advancements in medical technology and the integration of digital health solutions contribute to the market’s expansion. Regulatory requirements for stringent post-market surveillance and vigilance are also fueling the market’s growth.
Region Insights
North America dominates the medical devices vigilance market due to its robust healthcare infrastructure, advanced medical device industry, and strong regulatory frameworks. Europe follows closely, with strict regulatory requirements and established healthcare systems. The Asia-Pacific region is expected to experience significant growth due to rising healthcare investments, increasing adoption of medical devices, and a large patient population.
Medical Devices Vigilance Market Scope
| Report Coverage |
Details |
| Growth Rate from 2024 to 2033 |
CAGR of 8.19% |
| Global Market Size in 2023 |
USD 80.40 Billion |
| Global Market Size in 2024 |
USD 86.99 Billion |
| Global Market Size by 2033 |
USD 176.80 Billion |
| Largest Market |
North America |
| Base Year |
2023 |
| Forecast Period |
2024 to 2033 |
| Segments Covered |
By Delivery Mode, By Application, and By End-user |
| Regions Covered |
North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Medical Devices Vigilance Market Dynamics
Drivers
Key drivers of the medical devices vigilance market include the increasing complexity of medical devices, the need for continuous monitoring of device performance, and the desire to prevent adverse events. Technological advancements in data collection and analysis tools also enable more efficient vigilance activities, facilitating timely identification and resolution of potential issues.
Opportunities
Opportunities in the medical devices vigilance market include the integration of artificial intelligence and machine learning to enhance data analysis and prediction capabilities. Additionally, collaboration between regulatory agencies, manufacturers, and healthcare providers can lead to more efficient vigilance processes. Expansion into emerging markets presents further opportunities for growth.
Challenges
Challenges in the medical devices vigilance market include the need for standardized reporting and data management practices across regions and the complexity of managing large volumes of data from various sources. Ensuring data privacy and security while maintaining transparency can also be challenging. Additionally, staying up-to-date with changing regulatory requirements and managing compliance can pose difficulties for market players.
Read Also: Tire Pyrolysis Oil Market Size to Reach USD 567.90 Mn by 2033
Medical Devices Vigilance Market Recent Developments
- In June 2022, Italy instituted substantial changes in national regulations on medical device vigilance in accordance with the procedures by European Regulations. 2017/475 for medical devices and 2017/476 for in vitro diagnostics.
Medical Devices Vigilance Market Companies
- ZEINCRO
- AssurX Inc.
- Sparta System
- Oracle Corporation
- Xybion Corporation
- Sarjen Systems Pvt. Ltd.
- MDI Consultants, Inc.
- AB-Cube
- Laerdal Medical.
- Omnify Software, Inc.
Segments Covered in the Report
By Delivery Mode
By Application
- Diagnostics
- Therapeutics
- Surgical
- Research
By End-user
- Clinical Research Organizations (CROs)
- Business Process Outsourcing (BPO)
- Original Equipment Manufacturers (OEM)
- Other End-users
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/