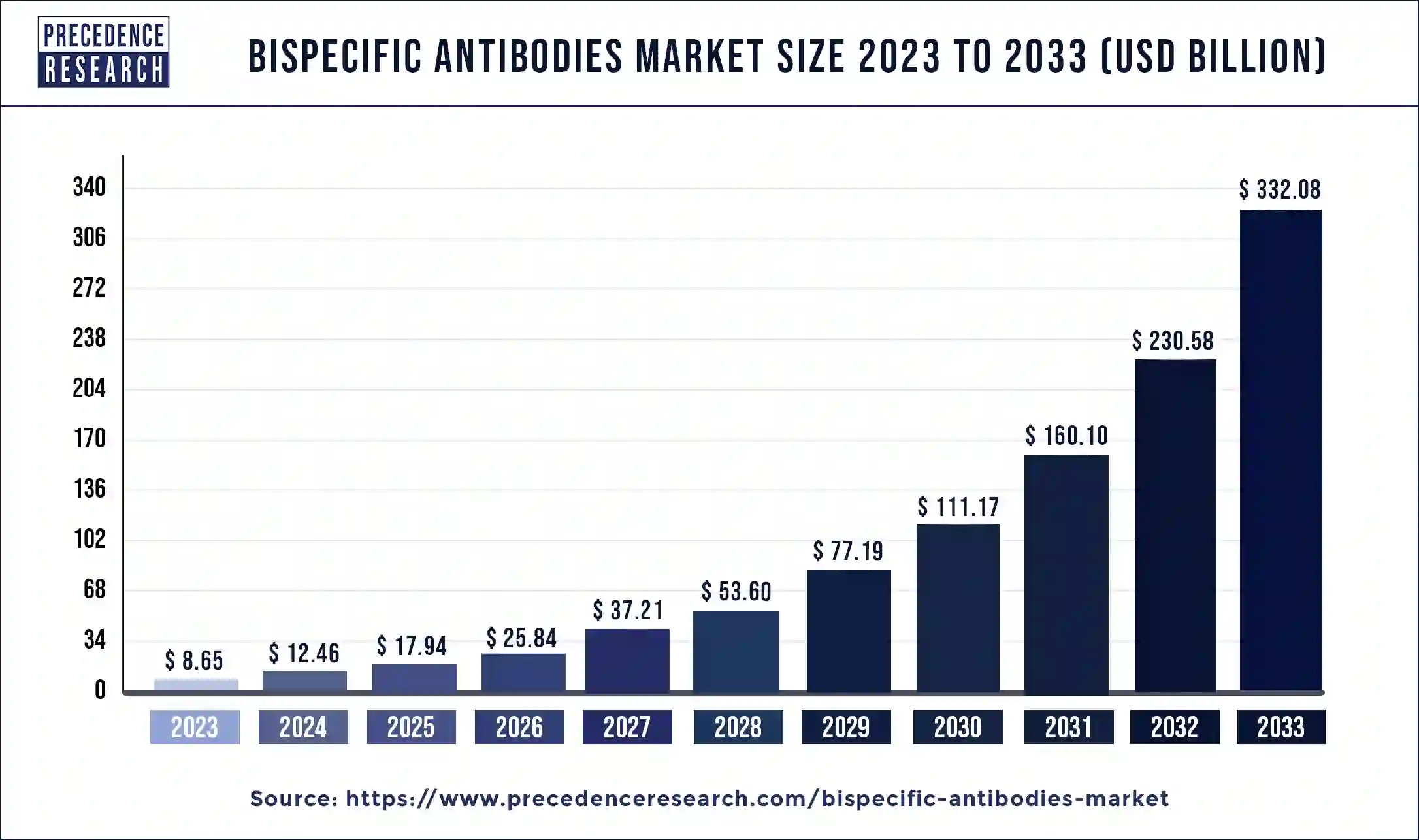
The global bispecific antibodies market size reached USD 8.65 billion in 2023 and is predicted to hit around USD 332.08 billion by 2033, expanding at a CAGR of 44.02% from 2024 to 2033.
Key Points
- North America has contributed more than 88% of the market share in 2023.
- Europe is estimated to expand at the fastest CAGR between 2024 and 2033.
- By indication, the cancer segment held the highest market share in 2023.
- By indication, the inflammatory and autoimmune disorders segment is anticipated to witness rapid growth at a significant CAGR during the projected period.
- By distribution channel, the hospital pharmacies segment has held the biggest market share in 2023.
- By distribution channel, the retail pharmacies segment is anticipated to witness significant growth over the projected period.
The bispecific antibodies market is experiencing significant growth globally due to their potential to revolutionize therapeutic approaches in various disease areas. Bispecific antibodies are engineered molecules that can simultaneously target two different antigens, offering advantages over conventional monoclonal antibodies. These molecules hold promise in treating cancer, autoimmune diseases, and infectious diseases by enhancing specificity, efficacy, and safety profiles. With ongoing advancements in biotechnology and increasing research investments, the bispecific antibodies market is poised for substantial expansion in the coming years.
Get a Sample: https://www.precedenceresearch.com/sample/3977
Growth Factors:
Several factors contribute to the robust growth of the bispecific antibodies market. One key driver is the rising prevalence of chronic diseases such as cancer and autoimmune disorders. Bispecific antibodies offer a novel therapeutic approach by targeting multiple disease pathways simultaneously, potentially leading to improved treatment outcomes. Additionally, the growing demand for personalized medicine and targeted therapies further fuels the adoption of bispecific antibodies, as they enable more precise and tailored treatment strategies.
Moreover, advancements in antibody engineering technologies have accelerated the development and production of bispecific antibodies. Innovations such as bispecific T cell engagers (BiTEs) and dual targeting antibodies have expanded the therapeutic capabilities of bispecific antibodies, enhancing their efficacy and versatility. Furthermore, strategic collaborations between pharmaceutical companies and research institutions have facilitated the discovery and development of novel bispecific antibody candidates, driving market growth through expanded pipelines and accelerated clinical trials.
Region Insights:
The bispecific antibodies market exhibits a global presence, with North America, Europe, Asia-Pacific, and the rest of the world representing key regions of interest. North America dominates the market due to the presence of a robust biopharmaceutical industry, favorable regulatory environment, and high healthcare expenditure. The region is also characterized by a strong focus on innovation and research, fostering the development and commercialization of novel bispecific antibody therapies.
In Europe, increasing investments in biotechnology and healthcare infrastructure support market growth. The presence of prominent biopharmaceutical companies and academic research institutions contributes to the region’s leadership in bispecific antibody research and development. Additionally, favorable reimbursement policies and expanding patient access to advanced therapies drive market expansion across European countries.
Asia-Pacific emerges as a rapidly growing market for bispecific antibodies, propelled by the increasing prevalence of chronic diseases and rising healthcare investments in countries such as China, Japan, and India. The region’s growing biotechnology sector, coupled with government initiatives to promote innovation and drug development, fosters market growth opportunities. Moreover, partnerships between regional and international pharmaceutical companies facilitate technology transfer and accelerate the commercialization of bispecific antibody therapies in Asia-Pacific markets.
Bispecific Antibodies Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 44.02% |
| Global Market Size in 2023 | USD 8.65 Billion |
| Global Market Size by 2033 | USD 332.08 Billion |
| U.S. Market Size in 2023 | USD 5.33 Billion |
| U.S. Market Size by 2033 | USD 206.02 Billion |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Indication and By Distribution Channel |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Bispecific Antibodies Dynamics
Drivers:
Several factors drive the adoption and utilization of bispecific antibodies in the healthcare industry. One of the primary drivers is the demand for more effective and targeted therapeutic interventions, particularly in oncology. Bispecific antibodies enable precise targeting of tumor cells while minimizing off-target effects, thereby improving treatment outcomes and patient survival rates. Additionally, the potential for bispecific antibodies to overcome resistance mechanisms observed with conventional therapies further enhances their attractiveness in cancer treatment.
Furthermore, the versatility of bispecific antibodies extends beyond oncology to other disease areas such as autoimmune disorders and infectious diseases. By modulating immune responses and targeting specific disease pathways, bispecific antibodies offer promising therapeutic solutions for conditions with significant unmet medical needs. The ability to engage multiple targets simultaneously enhances the efficacy of bispecific antibodies, making them valuable assets in addressing complex diseases with heterogeneous pathologies.
Moreover, advancements in antibody engineering technologies and manufacturing processes have contributed to the development of bispecific antibodies with improved pharmacokinetic properties and reduced immunogenicity. Enhanced stability, prolonged half-life, and reduced production costs increase the feasibility of bispecific antibody therapeutics, driving their adoption in clinical practice. Additionally, regulatory agencies’ increasing acceptance of bispecific antibody-based therapies accelerates their translation from preclinical research to clinical development and commercialization, further fueling market growth.
Opportunities:
The bispecific antibodies market presents numerous opportunities for innovation and expansion. One significant opportunity lies in the development of novel bispecific antibody formats and platforms with enhanced therapeutic properties. By leveraging emerging technologies such as antibody engineering, protein design, and computational modeling, researchers can design next-generation bispecific antibodies with improved efficacy, specificity, and safety profiles.
Furthermore, the exploration of combination therapies involving bispecific antibodies holds promise for synergistic treatment effects and improved patient outcomes. Combinatorial approaches, such as combining bispecific antibodies with immune checkpoint inhibitors or targeted small molecules, offer potential synergies in overcoming treatment resistance and enhancing therapeutic responses. Additionally, the identification of novel disease targets and biomarkers presents opportunities for the development of targeted bispecific antibody therapies across various disease indications.
Moreover, expanding market access and commercialization efforts in emerging economies represent a significant opportunity for bispecific antibody manufacturers. By addressing unmet medical needs and partnering with local stakeholders, companies can tap into growing patient populations and leverage market expansion opportunities in regions with evolving healthcare infrastructures. Strategic collaborations, licensing agreements, and joint ventures also offer avenues for accessing new markets and strengthening global market presence.
Challenges:
Despite the promising growth prospects, the bispecific antibodies market faces several challenges that could impede its trajectory. One such challenge is the complexity and variability associated with bispecific antibody development and manufacturing. Designing bispecific antibodies with optimal binding affinity, stability, and pharmacokinetic properties requires sophisticated engineering approaches and rigorous optimization, leading to prolonged development timelines and increased development costs.
Additionally, regulatory challenges surrounding the approval and commercialization of bispecific antibody therapeutics present hurdles for market entry. Regulatory agencies often require comprehensive preclinical and clinical data to evaluate the safety, efficacy, and quality of bispecific antibodies, necessitating extensive resources and expertise for successful regulatory submissions. Variability in regulatory requirements across different geographic regions further complicates the global development and commercialization of bispecific antibody products.
Furthermore, intellectual property (IP) considerations and patent disputes pose challenges to market competition and innovation within the bispecific antibodies landscape. Companies must navigate complex IP landscapes and engage in strategic patent protection strategies to safeguard their proprietary technologies and product pipelines. Patent litigation and challenges from competitors may result in market exclusivity uncertainties and impact companies’ investment decisions and market positioning.
Moreover, market access and reimbursement dynamics present challenges for the widespread adoption of bispecific antibody therapies, particularly in healthcare systems with cost containment measures and reimbursement constraints. Demonstrating the value proposition of bispecific antibodies compared to existing treatment modalities requires robust health economic evidence and stakeholder engagement to secure favorable reimbursement decisions and market access pathways.
Read Also: Vitamin K Market Size to Reach USD 2,126.08 Million by 2033
Recent Developments
- In January 2023, the FDA granted approval to Genentech for bispecific antibodies intended for lymphoma treatment.
- In July 2022, Janssen Pharmaceutical announced the receipt of conditional marketing authorization for Teclistamab in multiple myeloma patients from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP). Additionally, the company has submitted a Biologics License Application (BLA) to the US FDA seeking clearance for its innovative medications.
Bispecific Antibodies Market Companies
- Adimab, Innovent Biologics, Inc (U.S.)
- Affimed GmbH (China)
- Amgen Inc (Germany)
- AstraZeneca (U.K.)
- Xencor (U.S.)
- Sanofi (France)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Regeneron Pharmaceuticals Inc (U.S.)
- Eli Lilly (U.S.)
- Pieris Pharmaceuticals, Inc (U.S.)
- Mereo BioPharma Group plc (U.K)
- Sobi, TG Therapeutics Inc (Swden)
- Merus (Netherlands)
- MacroGenics, Inc (U.S.)
- Genmab A/S (Denmark)
- Emergent BioSolutions Inc (U.S.)
- Alteogen (South Korea)
- Astellas Pharma Inc (Japan)
- Novartis AG (Switzerland)
- CELGENE CORPORATION (U.S.)
Segments Covered in the Report
By Indication
- Cancer
- Inflammatory & Autoimmune Disorder
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Drugstores
- Online Pharmacies
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.uswebwire.com/
