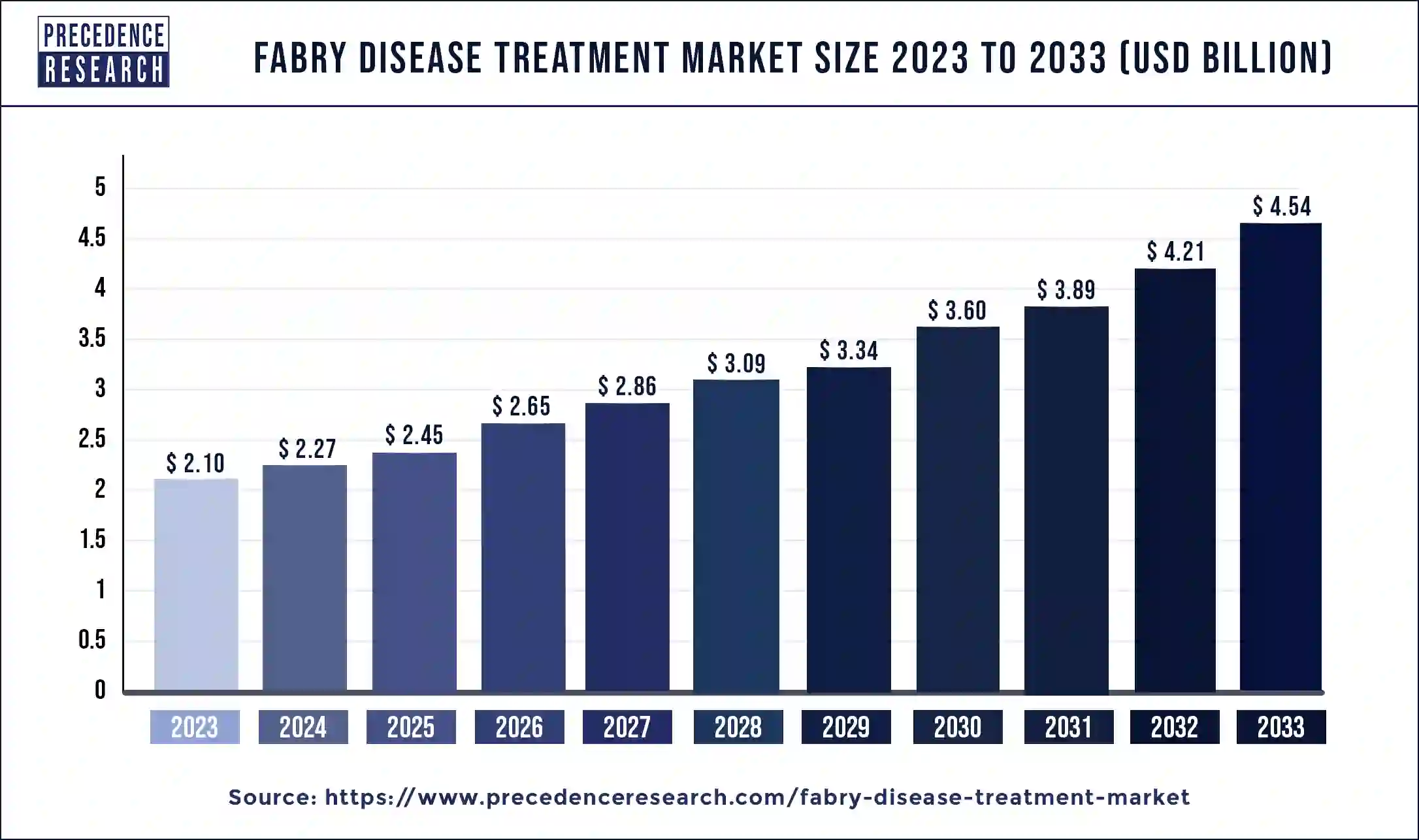
The global fabry disease treatment market size was exhibited at USD 2.10billion in 2023 and is anticipated to be expanding around USD 4.54 Billion By 2033, growing at a impressive CAGR of 8.02% from 2024 to 2033
Key Points
- The North America fabry disease treatment market size accounted for USD 710 million in 2023 and is expected to attain around USD 1,570 million by 2033, poised to grow at a CAGR of 8.25% between 2024 and 2033.
- North America dominated the market in 2023.
- Asia Pacific is expected to host the fastest-growing market over the forecast period.
- By treatment, the enzyme replacement therapy segment dominated the market in 2023.
- By treatment, the substrate reduction therapy segment grows at a rapid pace in the market over the forecast period.
Market Overview
Fabry Disease is a rare genetic disorder caused by the buildup of a particular type of fat, called globotriaosylceramide, in the body’s cells. This accumulation occurs due to the deficiency of the enzyme alpha-galactosidase A. The Fabry Disease Treatment Market focuses on therapies designed to manage the symptoms and progression of this condition. Treatments include enzyme replacement therapy (ERT), chaperone therapy, gene therapy, and substrate reduction therapy (SRT). The market for Fabry disease treatment is experiencing growth due to advancements in medical research and increased awareness about rare diseases.
Get Sample Copy of This Report@ https://www.precedenceresearch.com/sample/4404
Growth Factors
Several factors contribute to the growth of the Fabry Disease Treatment Market. Firstly, advancements in biotechnology and medical research have led to the development of innovative therapies, including gene therapy, which holds promise for long-term disease management. Additionally, increasing awareness about rare diseases has spurred early diagnosis and treatment, which in turn drives demand for effective therapies. Supportive government policies and funding for rare disease research also play a crucial role in market expansion. Furthermore, collaborations between pharmaceutical companies and research institutes accelerate the development and commercialization of new treatments.
Fabry Disease Treatment Market Scope
| Report Coverage | Details |
| Fabry Disease Treatment Market Size in 2023 | USD 2.10 Billion |
| Fabry Disease Treatment Market Size in 2024 | USD 2.27 Billion |
| Fabry Disease Treatment Market Size by 2033 | USD 4.54 Billion |
| Fabry Disease Treatment Market Growth Rate | CAGR of 8.02% from 2024 to 2033 |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Key drivers of the Fabry Disease Treatment Market include the increasing prevalence of Fabry disease and related disorders, which necessitates effective treatment options. The development of novel therapies and the approval of new drugs by regulatory authorities boost market growth. Moreover, patient advocacy groups and rare disease organizations play a vital role in raising awareness, which leads to early diagnosis and treatment. Technological advancements in genomics and biotechnology also drive the market by enabling the development of more effective and targeted therapies.
Opportunities
The Fabry Disease Treatment Market presents several opportunities for growth and innovation. The ongoing research in gene therapy offers potential for curative treatments, which can transform the management of Fabry disease. Additionally, there is an opportunity to develop personalized medicine approaches, tailoring treatments to individual patient needs based on genetic and biomarker data. The expansion of healthcare infrastructure in emerging markets provides new avenues for market penetration. Collaborations and partnerships between biotech firms, pharmaceutical companies, and academic institutions can also foster the development of novel therapies and enhance market reach.
Challenges
Despite the positive outlook, the Fabry Disease Treatment Market faces several challenges. High treatment costs and limited access to advanced therapies in some regions can hinder market growth. Additionally, the rarity of the disease means that clinical trials often have small patient populations, making it difficult to gather robust data and achieve regulatory approval. There is also a need for increased awareness and education among healthcare providers to ensure early diagnosis and appropriate treatment. Furthermore, the long-term efficacy and safety of new therapies, particularly gene therapy, need to be established through extensive clinical research.
Region Insights
The Fabry Disease Treatment Market is segmented geographically into North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa. North America holds a significant share of the market due to advanced healthcare infrastructure, high healthcare expenditure, and the presence of leading pharmaceutical companies. Europe follows closely, with substantial investment in rare disease research and a strong regulatory framework supporting orphan drug development. The Asia-Pacific region is expected to witness the fastest growth, driven by increasing healthcare awareness, improving diagnostic facilities, and growing investment in the healthcare sector. Latin America and the Middle East & Africa are also emerging markets, with improving healthcare infrastructure and growing patient awareness contributing to market growth.
Read Also: https://www.expresswebwire.com/embolic-protection-devices-market/
Fabry Disease Treatment Market Recent Developments
- In May 2023, Chiesi Global Rare Diseases and Protalix BioTherapeutics, Inc. received FDA approval for Elfabrio (pegunigalsidase alfa-ix) in the United States for the treatment of adult patients with Fabry disease. Elfabrio is supplied as a preservative-free solution in a single-dose vial.
- In May 2023, Sangamo Therapeutics, Inc., a genomic medicine company, received Fast Track Designation from the FDA for isaralgagene civaparvovec, or ST-920, a wholly owned gene therapy product candidate for the treatment of Fabry disease. ST-920 is currently being evaluated in the Phase 1/2 STAAR study, with a total of 20 patients diagnosed to date.
- In May 2023, Europe allowed Chiesi Farmaceutici and Protalix BioTherapeutics to market their product PRX-102 (pegunigalsidase alfa) in the European territory as a treatment for Fabry disease.
- In September 2022, the agency granted the Orphan Drug Designation (ODD) to AL01211, produced by AceLink Therapeutics, for treating Fabry disease. It is a glucosylceramide synthase (GCS) inhibitor and has shown high potency. The treatment was claimed as a much-needed orally consumed medicine as compared to other available treatments.
Fabry Disease Treatment Market Companies
- JCR Pharmaceuticals
- Sanofi Genzyme
- Green Cross Corporation
- Chiesi Group
- Regenxbio Inc.
- Idorsia Pharmaceuticals
- Protalix BioTherapeutics
- Amicus Therapeutics
Segments Covered in the Report
By Treatment
- Enzyme Replacement Therapy (ERT)
- Chaperone Treatment
- Substrate Reduction Therapy (SRT)
- Others
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.dailystatsnews.com/
Blog: https://www.dailytechbulletin.com/
